X chromosome inactivation is a fascinating yet complex biological phenomenon that plays a crucial role in gene expression, especially in females who possess two X chromosomes. This essential process ensures that only one X chromosome remains active while the other is effectively silenced, balancing gene dosage between sexes. The implications of X chromosome inactivation extend beyond mere genetics; it has significant ties to genetic disorders such as Fragile X Syndrome and Rett Syndrome, where mutations can disrupt normal cellular functions. Recent studies have shed light on chromosomal silencing mechanisms, revealing potential avenues for innovative gene therapy solutions aimed at these disorders. As researchers like Jeannie T. Lee explore this realm, they pave the way for advancements in treating conditions linked to aberrations on the X chromosome, offering hope to countless individuals and families affected by these genetic challenges.
The inactivation of one of the two X chromosomes in females serves as a critical regulatory mechanism in genetic expression, contributing to cellular homeostasis. This intricate process, often referred to as X inactivation or X-linked gene silencing, is key to understanding various genetic conditions that emerge due to improper gene dosage. For instance, disorders like Fragile X Syndrome and Rett Syndrome highlight the impact of mutations on the X chromosome and the pursuit of therapeutic interventions through advancements in gene therapy. Exploring the dynamics of chromosomal silencing not only enhances our comprehension of genetic disorders but also illuminates potential strategies for alleviating the burdens they impose. As scientists continue to unlock the complexities of this subject, the promise of innovative treatments draws closer, offering new avenues for hope and healing.
The Role of X Chromosome Inactivation in Genetic Disorders
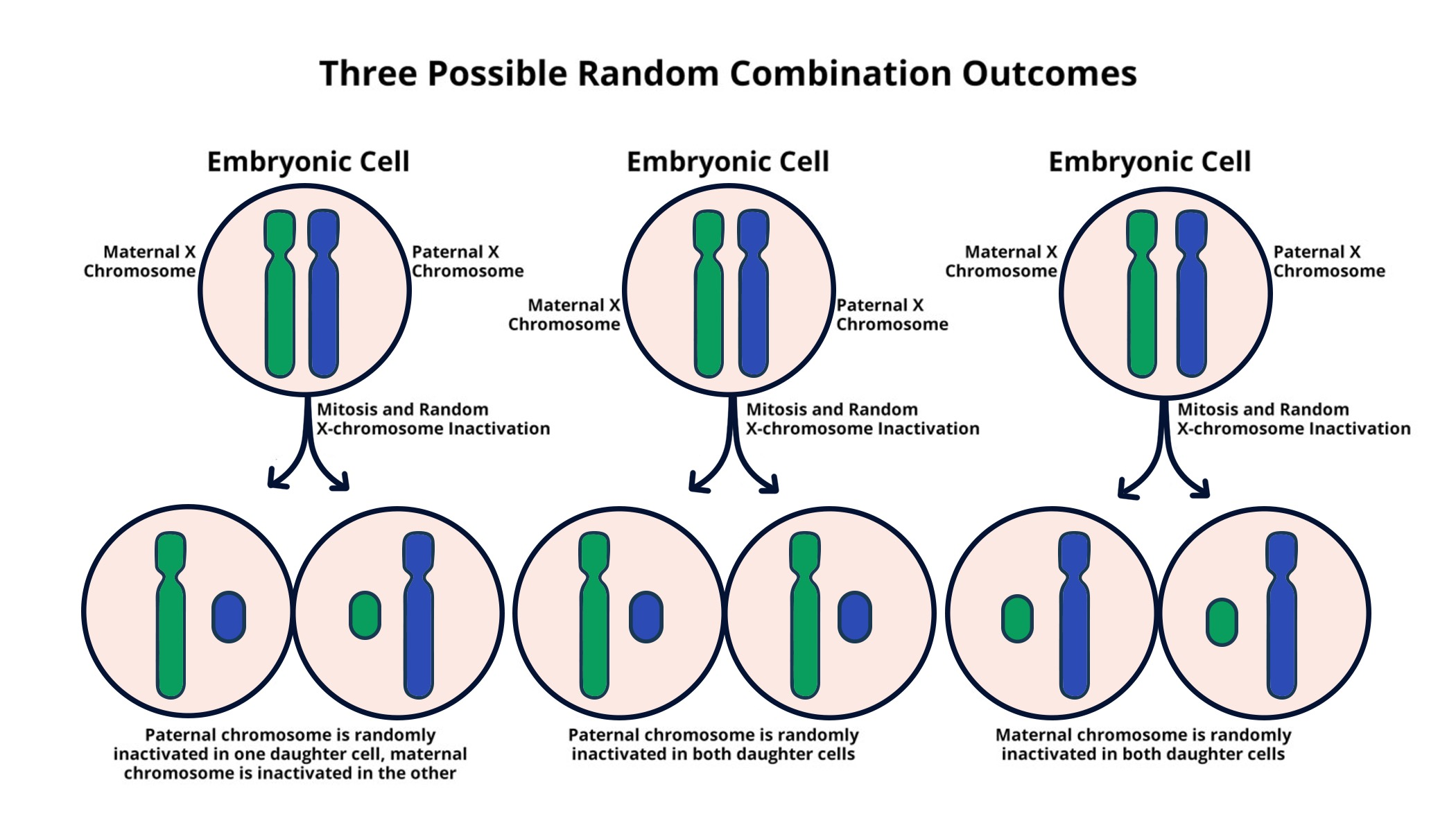
X chromosome inactivation (XCI) is a vital process in female mammals that ensures dosage compensation for genes located on the X chromosome. This means that during the development of an embryo, one of the two X chromosomes in females is randomly inactivated, allowing cells to maintain balance in gene expression between genders. Disruptions in this process can lead to various genetic disorders, notably Fragile X Syndrome and Rett Syndrome, both of which can result from abnormalities on the X chromosome. Understanding XCI can provide insights into how these conditions arise and how they might be treated.
Recent advancements in the study of XCI have illuminated the complexities of this process, particularly how a gelatinous substance enables chromosomal silencing. This biophysical change allows cells to utilize proteins that could otherwise be obstructed by the inactivated X chromosome. Consequently, therapies targeting XCI hold promise for treating conditions that stem from X-linked mutations. For instance, the ability to ‘unsilence’ inactivated X-linked genes could potentially pave the way for innovative gene therapies aimed at providing relief to individuals suffering from these debilitating genetic disorders.
Recent Advances in Gene Therapy for Fragile X and Rett Syndromes
Research led by Jeannie Lee’s lab has recently made significant strides in the quest for effective treatments for Fragile X Syndrome and Rett Syndrome. By focusing on the mechanics of how cells inactivate one of the X chromosomes, researchers have developed methods to reverse this process in laboratory settings. This groundbreaking work involves manipulating the ‘Jell-O-like’ substance that surrounds chromosomes to allow the expression of previously silenced genes. The dual approach could potentially reintroduce functional copies of genes necessary for normal development and cognitive function.
Moreover, with small-scale successes in preclinical models, the need for clinical trials is becoming more pressing. These trials aim to assess the safety and efficacy of therapies targeting chromosomal silencing for patients with these syndromes. By leveraging gene therapy techniques, researchers hope to not only address the symptoms of Fragile X and Rett Syndromes—but also to correct the underlying genetic disturbances. Such initiatives represent a beacon of hope in the realm of genetic disorders, where current therapeutic options have remained limited.
Understanding Chromosomal Silencing Mechanisms
Chromosomal silencing is a fundamental mechanism in cellular biology, crucial for regulating gene expression and maintaining genomic stability. The recent discoveries about how X chromosome inactivation occurs contribute significantly to our understanding of both normal and abnormal genetic functioning. At the core of this process lies the RNA molecule Xist, which plays a critical role in initiating the inactivation of one X chromosome in females. By altering the properties of the surrounding chromosomal matrix, Xist enables the cells to ‘turn off’ the genes on the inactivated X, creating a balance between the X-linked genes in both sexes.
This knowledge not only uncovers the intricacies of gene regulation but also highlights the potential for therapeutic intervention in genetic disorders. As researchers delve deeper into the molecular underpinnings of chromosomal silencing, they are piecing together how these mechanisms might be harnessed to develop more effective treatments. For instance, gene therapies targeting X chromosomes could potentially unmask healthy genes previously rendered inactive, offering new avenues for reversing the effects of mutations that lead to conditions like Fragile X and Rett Syndromes.
The Potential of Reversing X Chromosome Inactivation
The idea of reversing X chromosome inactivation presents a promising frontier in the treatment of genetic disorders linked to the X chromosome. As mutations often reside on the active X chromosome, freeing the inactivated X presents a unique opportunity to allow the expression of healthy alleles. Research demonstrates that once specific genes are activated through this process, cells can begin to function normally, offering hope to patients afflicted with conditions like Fragile X Syndrome, characterized by cognitive impairments, and Rett Syndrome, with its profound impacts on neurodevelopment.
The journey towards realizing such therapies is complex, as researchers must ensure that the mechanisms of activating these genes do not disrupt healthy gene functions on the active X chromosome. The Lee lab’s commitment to uncovering the nuanced interactions of XCI brings a sense of optimism, as preliminary findings suggest that careful regulation may lead to significant therapeutic outcomes without severe side effects. As this field continues to evolve, the possibility of transforming treatment for many genetically-based conditions becomes increasingly plausible.
Chromosomal Research and Its Implications for Future Treatments
The prolonged research conducted by Jeannie Lee and her team has shifted the landscape of chromosomal biology towards addressing critical health challenges posed by genetic disorders. The intricate study of X chromosome inactivation not only furthers our understanding of normal cellular processes but also opens doors to potential treatments for diseases such as Fragile X and Rett Syndromes. This foundational knowledge serves as the bedrock for developing innovative therapies aimed at correcting gene expression imbalances that lead to these disorders.
Ultimately, the commitment to enhancing our understanding of chromosomal dynamics has profound implications not just for therapeutic strategies, but for genetic counseling and risk assessment. As more genetic disorders linked to X chromosome mutations are identified, this research could guide the development of targeted interventions. In the future, individuals impacted by these disorders may benefit from treatments grounded in solid scientific discoveries about chromosomal silencing mechanisms and their applications in gene therapy.
The Intersection of X Chromosome Inactivation and Genetic Disorders
The intricate relationship between X chromosome inactivation and genetic disorders is a critical area of study, particularly concerning conditions like Fragile X Syndrome and Rett Syndrome. These disorders exemplify the consequences of improper gene regulation, stemming from the effects of mutations on the X chromosome. In females, the random inactivation of one X chromosome can conceal these anomalies, yet they remain a significant risk for both genders, given that males possess a single X chromosome susceptible to the same mutations.
Understanding how X chromosome inactivation and subsequent gene expression contribute to these disorders illuminates the pathway to potential therapies. Recent findings indicate that while one gene may be silenced, the healthy version can still be accessed if the inactivation is lifted—presenting a unique opportunity for treatments. This interconnectedness emphasizes the need for ongoing research into chromosomal mechanisms as we refine approaches to genetic disorders, ultimately aiming for universal benefits.
Potential of Chromosomal Silencing in Innovative Therapies
The therapeutic potential of targeting chromosomal silencing cannot be overstated, especially in the context of pervasive genetic disorders such as Fragile X and Rett Syndromes. By successfully understanding and manipulating the inactivation of the X chromosome, researchers can potentially develop innovative therapies that would allow the expression of previously silenced genes. This could lead to meaningful improvements in the quality of life and functional abilities of individuals diagnosed with these conditions.
As research progresses, the exploration of chromosomal silencing opens avenues for treating a broader range of genetic disorders beyond X-linked conditions. The mechanisms driving inactivation could provide critical insights into l interventions for various genetic diseases, promoting a multifaceted approach to gene therapy. By innovating and optimizing methods to reverse inactivation, the future of genetic disorder treatment may witness groundbreaking advancements with far-reaching implications.
The Future of X Chromosome Research in Gene Therapy
Exploring the future of X chromosome research through the lens of gene therapy brings hope for developing cures for a host of genetic disorders. The foundational work done by Jeannie Lee and her team provides a rich tapestry of knowledge that other researchers can build upon. By understanding how chromosomal silencing occurs and developing methods to reverse this process, we stand on the brink of transformative therapeutics that can address conditions arising from X-linked gene mutations.
The promise of clinical trials on the horizon suggests that we may soon see the fruits of this labor yield tangible benefits for those experiencing the challenges of Fragile X and Rett Syndromes. Innovations in gene therapy rooted in advancements in chromosomal biology not only inspire hope for patients but also incentivize further exploration into other genetic disorders. As our grasp of these biological processes deepens, the potential for novel treatments continues to expand, shaping the future of medical research.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation is a biological process that occurs in females where one of the two X chromosomes is silenced to balance gene dosage between males and females. This chromosomal silencing is crucial in preventing genetic disorders caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome, since it allows for the expression of healthy genes from the active X chromosome.
How is X chromosome inactivation related to Fragile X Syndrome and Rett Syndrome?
Fragile X Syndrome and Rett Syndrome are both genetic disorders linked to mutations on the X chromosome. X chromosome inactivation plays a significant role in these disorders as it can prevent the expression of healthy genes, making affected individuals reliant on the dysfunctional genes. The research into unsilencing these inactivated genes holds promise for potential gene therapy treatments.
What role does the Xist RNA molecule play in X chromosome inactivation?
Xist, or X-inactive specific transcript, is a key RNA molecule that initiates X chromosome inactivation. It alters the physical properties of the chromosomal silencing substance, facilitating the coating of one X chromosome in females. This process effectively leads to the inactivation of genes on that chromosome and may influence therapeutic strategies for certain genetic disorders.
Can X chromosome inactivation be targeted for gene therapy in treating Fragile X and Rett Syndromes?
Yes, targeting X chromosome inactivation presents a therapeutic opportunity for treating genetic disorders like Fragile X Syndrome and Rett Syndrome. By freeing inactivated X chromosomes, researchers can potentially restore the function of healthy genes that are normally silenced due to inactivation, allowing for the treatment of conditions caused by mutations.
What are the implications of chromosomal silencing on gene expression for males with X-linked disorders?
While males only have one X chromosome and do not undergo X chromosome inactivation, chromosomal silencing can still affect gene expression related to X-linked disorders. For example, mutations on the X chromosome like those responsible for Fragile X Syndrome can be selectively silenced, complicating gene expression. Understanding this process provides insights into potential treatments for affected males.
How does the Jell-O-like substance affect X chromosome inactivation?
The Jell-O-like substance, as described in recent research, surrounds chromosomes and facilitates chromosomal organization. In X chromosome inactivation, this gelatinous material is modified by Xist RNA, allowing the chromosome to become more flexible, which aids in the inactivation process. This discovery can lead to advances in understanding and potentially correcting genetic disorders.
What advances have been made in unsilencing X-linked genes for potential therapies?
Recent advances in the lab of Jeannie T. Lee involve developing methods to unsilence X-linked genes in isolated cells. These methods aim to retrieve healthy gene function in conditions like Fragile X Syndrome and Rett Syndrome, opening avenues for new treatments. Future clinical trials may test these promising therapies on individuals with these conditions.
Why is understanding X chromosome inactivation crucial for advancements in genetic research?
Understanding X chromosome inactivation is pivotal as it provides insights into the mechanisms of gene expression regulation on the X chromosome. This knowledge is essential for developing targeted gene therapies for X-linked genetic disorders, which could significantly improve treatment options and outcomes for affected individuals.
| Key Points |
|---|
| The X chromosome presents a unique challenge as females have two copies while males possess only one. |
| Females inactivate one X chromosome to avoid having double the dosage of genes encoded on it. |
| Jeannie Lee’s lab has significantly contributed to understanding X chromosome inactivation mechanisms. |
| A gelatinous substance acts as a medium that separates chromosomes and helps in X chromosome inactivation. |
| The gene Xist instructs cells to change the properties of this gelatinous substance. |
| Xist helps in the inactivation of the X chromosome by altering the viscosity of the surrounding material. |
| Research by Lee may lead to therapeutic applications for diseases caused by mutations on the X chromosome, including Fragile X and Rett syndromes. |
| Efforts are underway to develop potential treatments that could free inactivated genes. |
| Unexpectedly, freeing inactivated genes tends to restore function to mutated genes without affecting healthy ones. |
| The research has been supported over decades by the National Institutes of Health. |
Summary
X chromosome inactivation is a crucial process that allows female cells to manage their dual X chromosomes effectively. By inactivating one of the X chromosomes, females can prevent an excess of gene dosage, maintaining cellular balance. The groundbreaking work by Jeannie T. Lee’s lab sheds light on the intricate mechanisms behind X chromosome inactivation, where molecules like Xist manipulate the surrounding gelatinous compartment to silence the chromosome. This research not only enhances our understanding of cellular biology but also opens doors to potential treatments for genetic disorders such as Fragile X and Rett syndromes by unsilencing mutated genes, thus offering hope for effective therapies.